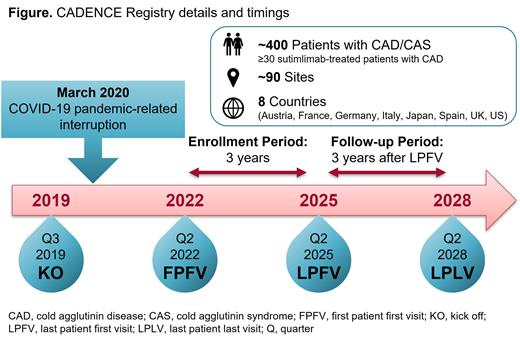
Cold agglutinin disease (CAD) is a rare chronic autoimmune hemolytic anemia (AIHA) characterized by classical complement pathway (CP)-mediated hemolysis. CAD is associated with a substantial disease burden negatively impacting quality of life (QoL), including severe fatigue, and increased risk of both thromboembolic events and early mortality. Due to the rarity of CAD, there is scarce information regarding its natural history, long-term impacts, and complications. Sutimlimab, a humanized monoclonal antibody that selectively inhibits the CP, is the first approved treatment for CAD. In the CARDINAL and CADENZA Phase 3 trials, sutimlimab reduced hemolysis, anemia, was generally well tolerated, and meaningfully improved QoL. The CADENCE Registry (NCT05791708) is a large international database of patients with CAD or cold agglutinin syndrome (CAS, a cold antibody-driven hemolytic anemia associated with an underlying condition). First launched in 2019, the first study patient was enrolled in 2022 following a COVID-19 pandemic-related hiatus. Presented here is an update on the current CADENCE protocol and study design.
CADENCE is a multinational, multicenter, observational, prospective, longitudinal registry study enrolling patients ≥18 years of age diagnosed with CAD (diagnostic criteria: a monospecific direct antiglobulin test strongly positive for C3d and negative or weakly positive for immunoglobulin G and a cold agglutinin titer ≥1:64) or CAS (CAD diagnostic criteria, with causative infection, autoimmune disorder or overt malignancy). Exclusion criteria include patients with a diagnosis of warm or mixed AIHA and those actively participating in a CAD/CAS interventional trial. The main study objective is to better understand patient demographics, clinical characteristics, treatment patterns, healthcare resource utilization (HRU), natural history of disease, long-term clinical outcomes, and impact on patient QoL. A sutimlimab-treated cohort sub-study is ongoing to assess the real-world safety and effectiveness of sutimlimab in patients with CAD, fulfilling the regulatory commitment for a post-authorization safety study. The enrollment period is expected to last ~3 years and patients will be followed for 3 years after the last patient enrolled. Data collection will include both clinical assessments as per standard of care and patient-reported outcome (PRO) data collected at enrollment and at ~6-month interval follow-ups. Additional safety data will be collected for the sutimlimab cohort.
The CADENCE Registry's first patient enrolled (after study restart) was in Q2 2022, with last patient's first visit expected in Q2 2025 and last patient's last visit expected in 2028 ( Figure). The registry will collect data on ~400 patients with CAD/CAS across ~90 sites in 8 countries: Austria, France, Germany, Italy, Japan, Spain, the UK, and the US. Among them, ≥30 patients with CAD treated with sutimlimab are expected to take part in the cohort study. Key data to be collected include clinical characteristics, disease complications, comorbidities, mortality, treatment regimens, HRU, and PROs. Biomarkers for hemolysis will be assessed, including lactate dehydrogenase, haptoglobin, total and indirect bilirubin. In the sutimlimab cohort, data will also be collected on adverse events (AEs), serious AEs, and AEs of special interest (serious infections, Raynaud's, arterial hypertension, autoimmune disorders, hypersensitivity/anaphylaxis, exposure in pregnancy, overdose). PRO measures include the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, the 36-item Short Form Health Survey (SF-36), and the CAD symptoms and impact questionnaire (CAD-SIQ), a novel 11-item questionnaire specifically designed for CAD. Interim analyses will occur annually. Final analyses will be performed at study end both at regional and global levels. As of July 13, 2023, 47 of 82 targeted sites have been activated, and 113 of 405 targeted patients (CAD/CAS) have been enrolled, including 13 sutimlimab-treated patients.
CADENCE is the first global registry for patients with CAD and will help advance understanding of the natural history and disease process of this rare condition. This registry will also assess the long-term safety and effectiveness of sutimlimab in patients with CAD in a real-world setting, providing clinicians with data that may help improve treatment practices.
Disclosures
Roeth:Sanofi: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Biocryst: Consultancy, Honoraria; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Apellis Apellis Pharmaceuticals: Consultancy, Honoraria. Barcellini:Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jaeger:BMS, Novartis, Gilead, Miltenyi, Janssen and Roche: Honoraria; Innovative Medicines Initiative 2 Joint Undertaking: Research Funding. Ueda:SOBI: Consultancy, Honoraria; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Asahi Kase: Consultancy. Michel:Sanofi: Consultancy; Alexion: Consultancy; Sobi: Consultancy; UCB: Honoraria; argenx: Honoraria; Novartis: Consultancy. Hill:Amgen: Consultancy; Argenx: Consultancy; Gliknik: Consultancy; Incyte: Consultancy; Immunovant: Consultancy; Janssen: Consultancy; Novartis: Consultancy, Honoraria; Sanofi: Consultancy; Sobi: Consultancy. Srivastava:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Yoo:Sanofi: Current Employment, Current equity holder in publicly-traded company. Broome:Argenx: Honoraria; Alexion: Honoraria; Sanofi: Honoraria; Apellis: Honoraria.